Groundbreaking Study Reveals Immune Mechanisms Behind Autoimmune Pancreatitis – Could New Biomarkers Lead to Better Treatments?
2024-10-07
Author: Sarah
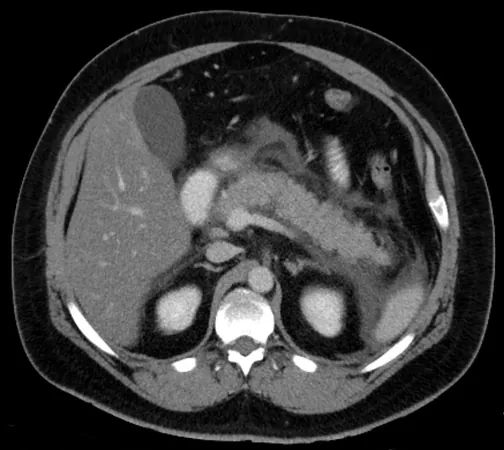
Understanding Autoimmune Pancreatitis (AIP)
Autoimmune pancreatitis (AIP) is a serious condition characterized by inflammation of the pancreas, resulting from the body’s abnormal sensitivity to its own proteins. This disease often goes hand-in-hand with other inflammatory conditions, such as autoimmune sialadenitis and cholangitis, collectively identified as IgG4-related disease (IgG4-RD). Recent experiments using murine models, particularly the MRL/MpJ mice, have underscored the similarities between these models and human AIP with IgG4-RD.
Role of Plasmacytoid Dendritic Cells in AIP
A closer examination of inflamed pancreatic tissues in these models revealed an abundance of plasmacytoid dendritic cells (pDCs) that produce signaling proteins like interferon-alpha (IFN-α) and interleukin-33 (IL-33). Notably, research indicated that the depletion of these pDCs or the inhibition of associated signaling pathways could prevent the onset of AIP, suggesting that activated pDCs play a crucial role in its development.
Unresolved Questions About pDCs and TLR3
Despite this progress, the mystery surrounding the origin of pDCs and their interaction with other immune cells such as effector T cells remained unresolved. Although pancreatic inflammation is initially triggered by a toll-like receptor 3 (TLR3) ligand, polyinosinic-polycytidylic acid (poly(I:C)), the low expression of TLR3 on pDCs further complicates understanding which cells truly initiate murine AIP. Moreover, while AIP promotes the creation of various T cell types, their role in advancing the disease still demands clarification.
Recent Research Findings
A recent study conducted by a team of esteemed researchers, including Associate Professor Tomohiro Watanabe from Kindai University, Dr. Akane Hara, Professor Masatoshi Kudo, and Dr. Warren Strober from the National Institute of Health in the U.S., addresses these questions. Their findings, published in JCI Insight, illuminated the immune signaling pathways crucial for initiating and sustaining AIP by investigating poly(I:C) induction in murine models.
According to Associate Professor Watanabe, "Our study identifies that poly(I:C) interacts with TLR3-bearing conventional dendritic cells (cDCs) to kickstart AIP in MRL/MpJ mice, leading to the production of IFN-α and IFN-β.” The researchers demonstrated that inhibiting TLR3 signaling significantly reduced the pancreatic inflammation typically seen at the onset of the disease; during this phase, cDCs were known to release inflammatory mediators including type I IFNs and chemokines such as CXCL9 and CXCL10, which facilitate the recruitment of CD4+CXCR3+ T cells to the pancreas.
Chemokine Interactions and pDC Migration
The team also explored the chemokine interactions enabling pDC migration into inflamed pancreatic tissue. They discovered that cell surface receptors on pDCs, like CCR2, CCR7, and CCR9, attract them to specific chemokines such as CCL2, CCL21, and CCL25. Notably, repeated poly(I:C) administration increased CCL2 and CCL25 levels, suggesting that interactions between these chemokines and their receptors may drive pDC migration into the pancreas.
Positive Feedback Loop in Inflammation
Moreover, the accumulation of pDCs triggers a positive feedback loop wherein they secrete CXCL9 and CXCL10, further attracting CXCR3+ Th1 cells which contribute to producing CCL25, hence recruiting more pDCs and perpetuating the inflammatory cycle.
Biomarkers for AIP and Future Directions
Previous investigations have pointed to serum levels of IFN-α and IL-33 as potential biomarkers for diagnosing AIP/IgG4-RD. This recent study corroborated these findings, establishing a positive correlation between serum concentrations of IgG4 and levels of IFN-α, IL-33, CXCL9, and CXCL10. Moreover, analyses of serum from patients with AIP/IgG4-RD mirrored elevated immunological markers observed in the murine model, although further research is warranted given the chronicity and multi-organ implications of AIP/IgG4-RD in humans.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)