Groundbreaking Universal Gene Therapy for Diamond-Blackfan Anemia Set for Clinical Trials
2024-11-26
Author: Siti
Groundbreaking Universal Gene Therapy for Diamond-Blackfan Anemia Set for Clinical Trials
In a remarkable breakthrough, researchers at Harvard Medical School have developed a universal gene therapy aimed at treating Diamond-Blackfan anemia (DBA), a rare but severe disorder characterized by the inability of the bone marrow to produce mature red blood cells. This significant advancement comes in the wake of challenges posed by the over 30 different genetic mutations that can trigger the disease.
The innovative therapy, which is ready to enter clinical trials, was announced in the prestigious journal Cell Stem Cell on November 11. Senior author Vijay Sankaran, the Jan Ellen Paradise, MD Professor of Pediatrics at Boston Children’s Hospital, emphasized the unprecedented nature of this achievement. “This is one of the first examples where we can develop a gene therapy that can target dozens of mutations with a single vector,” he stated.
Targeting the Root Cause: GATA1
DBA, first described in 1938 at Boston Children’s, offers limited treatment options. While some patients may benefit from bone marrow transplants if they have well-matched donors, most rely on steroid treatments, which can cause undesirable side effects, or frequent blood transfusions.
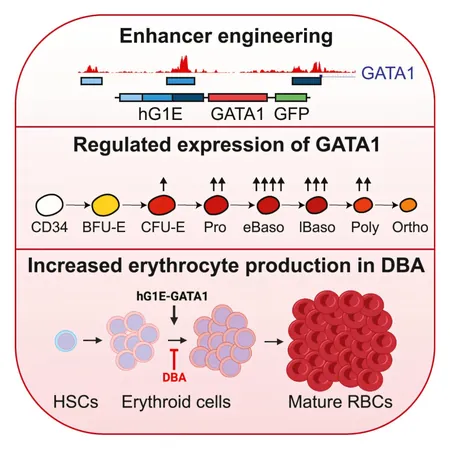
The various gene mutations linked to DBA were predominantly discovered at Boston Children's over the last 15 years by Sanakaran and colleague Hanna Gazda. These mutations primarily affect ribosomes—cellular structures crucial for protein synthesis. In a pivotal finding from 2012, Sankaran identified specific mutations in the GATA1 gene that regulate early red blood cell production. He established that ribosomal mutations inhibit the production of GATA1 protein, leading to the anemia observed in affected patients.
Further research illustrated that administering GATA1 protein to blood stem cells from DBA patients enabled these cells to differentiate more effectively into red blood cells. This opened the door for gene therapy targeting not only GATA1 mutations but also providing a solution for the ribosomal mutations that impede red blood cell production.
Innovative Gene Delivery System
To successfully deliver the GATA1 gene into patients, Sankaran's team engineered a non-infectious lentivirus as a vector. However, they faced a significant hurdle: the cells would differentiate into mature red blood cells too rapidly and wouldn’t integrate into the bone marrow. Through extensive research, the team discovered a mechanism to control the expression of the GATA1 gene, allowing it to be inserted into blood stem cells without prematurely prompting their differentiation.
Lab experiments revealed that this approach significantly enhanced the production of mature red blood cells while preserving the stem cells’ regenerative capabilities. Importantly, the therapy was designed to insert the GATA1 gene in a targeted fashion, minimizing risks associated with unintended insertions near oncogenes, making the therapy appear safe according to preliminary assessments by the researchers.
The team is currently submitting an Investigational New Drug application to the FDA, aiming to kickstart clinical trials.
Potential Broader Impact
Initial laboratory results show that this innovative gene therapy could substantially increase red blood cell production when compared to existing treatments. However, only a clinical trial will confirm the therapy's efficacy in real-world applications.
The potential societal impact is significant, especially in addressing racial and ethnic disparities in health caused by the difficulty of finding bone marrow donors for individuals suffering from DBA. If successful, this gene therapy could pave the way for similar approaches in treating various blood disorders.
Sankaran is optimistic about the broader implications of this work. He noted, “This work demonstrates for the first time how targeting the downstream mechanism rather than individual mutations could expand the reach of hematopoietic gene therapy.” This paradigm shift could open new avenues for tackling a host of complex blood diseases.
In conclusion, the advent of this universal gene therapy holds promise not only for Diamond-Blackfan anemia but also sets a precedent for future genetic treatments that may benefit countless others. Stay tuned as this groundbreaking research unfolds in clinical settings!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)