Revolutionary Breakthrough: Micro-Robots Utilizing Ultrasound for Targeted Drug Delivery to Tumors!
2024-12-12
Author: Yu
Introduction
In an astonishing development that could reshape the future of medicine, researchers are unveiling miniature robots designed to deliver therapeutic drugs directly to tumor sites in the body. Rather than traditional mechanical robots, these innovative tools take the form of tiny, bubble-like spheres that promise precision and effectiveness in targeting disease.
Overview of Bioresorbable Acoustic Microrobots (BAM)
These bioresorbable acoustic microrobots (BAM) not only survive harsh bodily environments, like stomach acid, but they are specifically engineered for controlled drug release at the exact location where it’s needed—your body's tumor sites. Recent experiments demonstrated their effectiveness in reducing bladder tumor sizes in laboratory mice, marking a significant milestone in medical technology.
Challenges in Micro-Robot Applications
Despite two decades of development, the application of micro- and nanorobots in patients has faced significant hurdles, primarily due to the complexities of navigating biological fluids such as blood and urine. Moreover, any robotic solution must be both biocompatible and bioresorbable, avoiding any toxic leftovers after treatment. The new spherical microrobots created by a dedicated team from the California Institute of Technology leverage poly(ethylene glycol) diacrylate—a hydrogel that safely retains fluids and is compatible with biological tissues.
Manufacturing Process
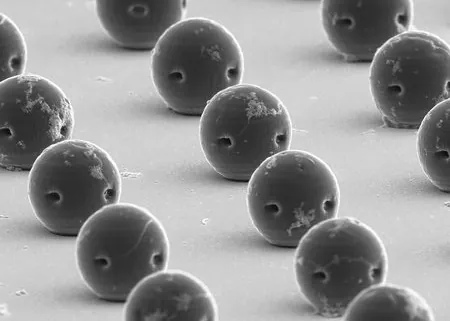
Manufacturing these microrobots employs a sophisticated process known as two-photon polymerization (TPP) lithography. This advanced technique utilizes focused infrared laser pulses to intricately link photosensitive polymers into a desired shape, layer by layer, akin to high-resolution 3D printing. Each microrobot measures around 30 microns in diameter—about the width of a human hair—and incorporates magnetic nanoparticles, which facilitate navigation within the body using an external magnetic field. When they reach the tumor site, the robots hold their position while releasing their therapeutic payload, as highlighted in the journal *Science Robotics*.
Design Features for Enhanced Functionality
To ensure optimal movement through bodily fluids, the team cleverly designed these microrobots with a hydrophilic (water-attracting) exterior to prevent clumping, while the interior is hydrophobic (water-repelling) to trap essential air bubbles. This unique construction allows the robots to remain mobile for extended periods, significantly enhancing their maneuverability and effectiveness. The two openings—one at the top and one on the side—further improve their speed in various viscous biofluids, demonstrating groundbreaking advances in micro-robotics.
Monitoring and Results
Tracking the movement of these microrobots is made possible through ultrasound imaging, thanks to the egg-shaped bubbles trapped within. These bubbles serve as excellent contrast agents for real-time monitoring. During testing, the microrobots were administered to mice with bladder tumors, showing remarkable success compared to conventional drug delivery methods. Over 21 days, four doses of medication effectively reduced tumors, showcasing the vast potential this technology holds for treating cancer and other diseases.
Future Prospects
"We believe this technology represents a pivotal moment in the future of drug delivery and precision surgery," stated Wei Gao, a professor of medical engineering at Caltech. "Our goal is to explore how these robots can deliver a variety of therapeutic agents for different medical conditions. In the long run, we anticipate this innovation could transition to human trials."
Conclusion
Stay tuned for more developments as this groundbreaking technology moves closer to providing targeted treatments that could revolutionize how we manage cancer and other health challenges in the years to come!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)