Revolutionary Drug Discovery: How "Twin" Proteins Are Changing Cancer Treatment!
2024-10-02
Introduction
Scientists at Scripps Research have made groundbreaking progress in cancer treatment by leveraging the concept of "twin" proteins, or paralogs, to identify new drug-binding sites. While some proteins are easily targeted with drugs due to their well-defined structures, others, particularly those involved in cancer and autoimmune diseases, present a significant challenge due to the absence of obvious drug-binding sites.
Paralog Hopping: A New Technique
In an innovative approach documented in a recent study published in *Nature Chemical Biology*, researchers pioneered a technique known as "paralog hopping." This method allows scientists to use the knowledge gained from one protein's paralog to develop drugs that can specifically inhibit its more elusive counterpart. Benjamin Cravatt, the senior author and a prominent figure in the field, remarked, "Being able to target one paralog while sparing another is a significant advancement. Different paralogs can perform distinct functions in the body, making selective targeting essential for effective drug development."
Focus on CCNE1 and CCNE2
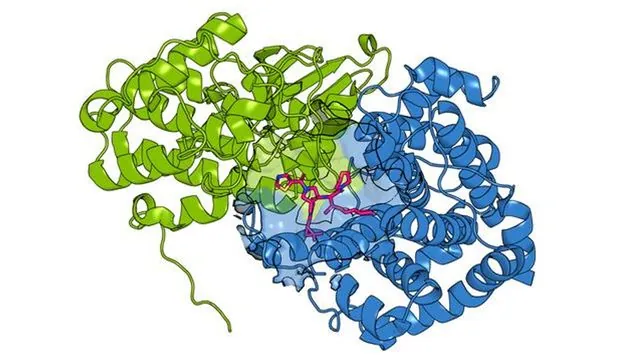
The study illuminates how gene duplication throughout evolution gives rise to these paralogs, which retain similar structures and functions. The researchers focused on the paralog pair CCNE1 and CCNE2, both implicated in breast, ovarian, and lung cancer. Remarkably, while CCNE2 contains a druggable cysteine—a prime target for drug binding—CCNE1 does not. The team's objective was to develop drugs that could still bind and inhibit CCNE1 despite its lack of an accessible cysteine site.
Engineering Cysteine into CCNE1
Under the leadership of graduate student Yuanjin Zhang, the team employed an innovative strategy: they engineered a cysteine into CCNE1 mirroring the drug-binding site of CCNE2. After identifying drugs that could bind to this modified CCNE1, they conducted high-throughput screening of various chemical compounds. Their results were astonishing: they discovered a number of compounds that could effectively bind to CCNE1 without relying on the cysteine, some of which did not affect CCNE2 at all. Others exhibited unique properties, capable of stabilizing the CCNE1 protein, thus enhancing its activity rather than inhibiting it.
Implications of the Discovery
This pivotal discovery established a new, previously unknown drug-binding pocket on CCNE1, prompting the researchers to emphasize the critical role of innovative drug screening methods. "If we had solely relied on traditional approaches focused on identifying compounds with specific functions, we would have missed a myriad of functional molecules," Zhang pointed out.
Future Directions
As a follow-up, the Scripps team plans to expand their paralog-hopping methodology to explore additional protein pairs that are crucial in cancer development. The potential impact of this research could pave the way for more nuanced and effective drugs targeting cancer, heralding a new era in cancer therapies.
Conclusion
Stay tuned as scientists continue to unravel the mysteries of protein interactions and unlock the keys to new and targeted cancer treatments!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)