Revolutionary Light-Activated Catalyst Boosts Chiral Synthesis: Higher Yields, Less Waste!
2025-04-10
Author: Siti
A Game-Changer in Chiral Chemistry
Researchers at the California Institute of Technology, in collaboration with experts from the University of Pittsburgh, have unleashed a groundbreaking light-activated catalyst that transforms the way chemists synthesize chiral molecules. Their recent study, featured in the prestigious journal Nature, unveils this novel catalyst's mechanisms and its promising applications.
The Enantiomer Challenge
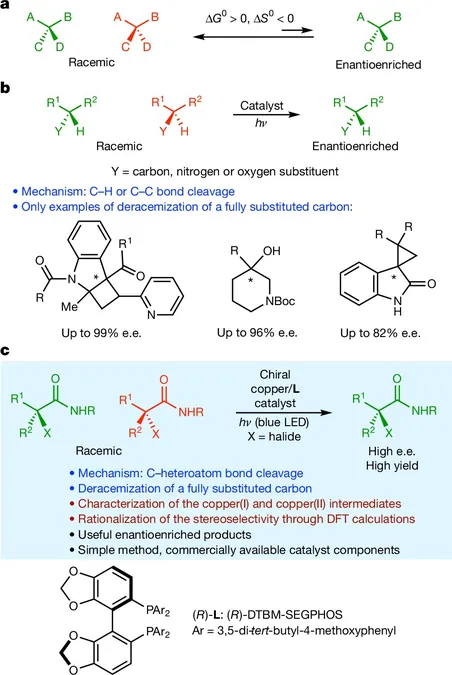
Chiral molecules exist as mirror-image pairs known as enantiomers, with only one being effective for most applications. Traditionally, chemists separate these isomers, discarding the less useful one—leading to significant waste. This inefficient process has driven scientists to seek a more sustainable solution.
Introducing the Light-Activated Catalyst
The Caltech team has made a remarkable stride by integrating a copper chloride with a chiral phosphine ligand that can tune its reactivity. Upon exposure to light, this catalyst ignites a transfer reaction between electrons and the halide substrate, effectively breaking their bonds and generating radical intermediates.
Streamlined Process with Stunning Results
In the subsequent reaction phase, chloride is transferred from the copper complex to the earlier formed radical, ultimately producing the desired single chiral enantiomer. This innovative method has demonstrated significantly higher yields compared to traditional separation techniques, showcasing its efficiency and potential for real-world applications.
A Bright Future for Green Chemistry
With this light-activated catalyst, chemists are not just enhancing the efficiency of chiral synthesis but also paving the way for greener practices in chemical production. The implications for pharmaceuticals and related fields could be immense, ushering in an era of reduced waste and optimized outcomes.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)