Revolutionary Machine Learning Breakthrough Predicts Gold Nanoparticle Interactions with Blood Proteins
2024-11-19
Author: Nur
Introduction
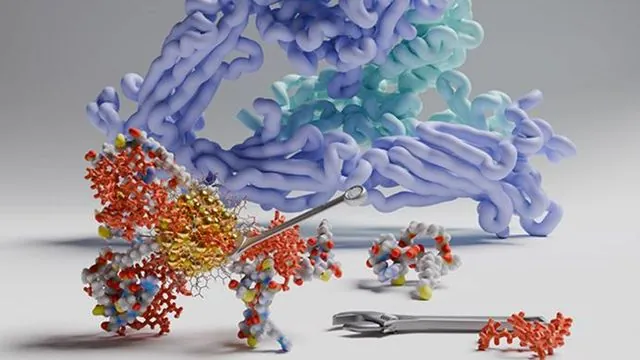
A groundbreaking study from researchers at the Nanoscience Center at the University of Jyväskylä, Finland, has unveiled a game-changing methodology utilizing machine learning and supercomputer simulations to predict how minuscule gold nanoparticles engage with blood proteins. This innovative approach not only allows scientists to foresee favorable interactions between nanoparticles and blood proteins but also paves the way for enhancing targeted drug delivery systems in the realm of precision nanomedicine.
Significance of Gold Nanoparticles
Gold nanoparticles, due to their unique properties, offer a wealth of potential in medical applications, ranging from drug delivery systems to cancer treatment. However, understanding the interactions at the nano-bio interface has remained a significant challenge for researchers. The complexity arises from crucial processes occurring across diverse time and length scales, influencing essential aspects like electronic charge transfer and biomolecule surface restructuring.
Machine Learning and Molecular Dynamics
The study's authors have made a remarkable advancement by employing machine learning driven by extensive molecular dynamics simulations of gold nanoparticle and protein systems in aquatic environments. Using graph theory and neural networks, the researchers could predict the most advantageous binding sites for nanoparticles on five prevalent human blood proteins, which include serum albumin, apolipoprotein E, immunoglobulin E, immunoglobulin G, and fibrinogen. The efficacy of these predictions was affirmed by extended atomistic simulations that mirrored these interactions.
Expert Insights
Professor Hannu Häkkinen, a prominent figure in computational nanoscience, stated, 'Our recent computational analysis demonstrated the possibility of selectively targeting over-expressed proteins on cancer cells with functionalized gold nanoparticles embedded with peptides and therapeutic drugs. The integration of our new machine learning techniques enables us to further explore how these drug-laden nanoparticles interact with blood proteins, consequently affecting drug carrier effectiveness.'
Future Directions
This research is set to continue evolving, as it lays the groundwork for the development of advanced computational methodologies focused on the interactions between metal nanoparticles and biomolecules. Professor Häkkinen expressed optimism for the future, declaring, 'Machine learning serves as a pivotal tool in examining nanoparticles in both diagnostic and therapeutic applications within nanomedicine. Our next endeavor, titled ‘Dynamic Nanocluster – Biomolecule Interfaces’, supported by the European Research Council, will aim to delve deeper into these vital interactions.'
Conclusion
As researchers expand their knowledge in this promising field, the implications of their findings could signal a new era of customized medical solutions utilizing nanoparticle technology, potentially transforming cancer therapies and diagnostics as we know them. Stay tuned for more updates on this fascinating research that could reshape healthcare!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)