Revolutionary Malaria Vaccine Administered by Mosquito Bite Shows Promising Results!
2024-11-21
Author: Daniel
Exciting Breakthrough in Malaria Vaccine Research
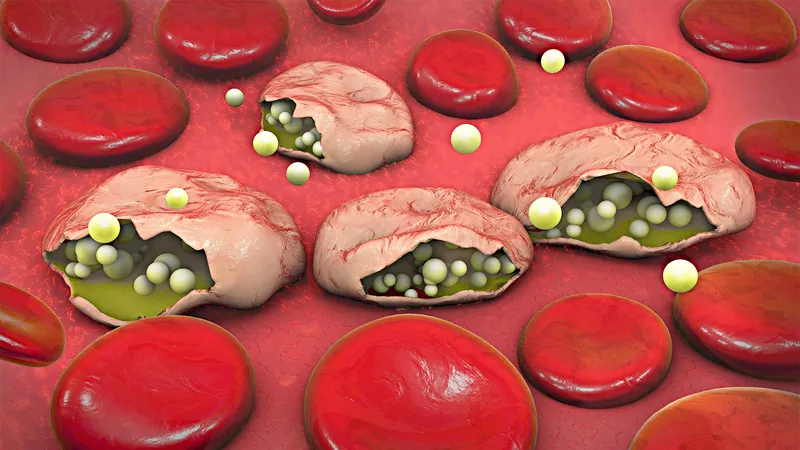
In an exciting breakthrough, a new malaria vaccine delivered through mosquito bites has demonstrated both safety and significant efficacy in a small clinical trial conducted in the Netherlands. Researchers at Leiden University Medical Center found that a second-generation genetically modified parasite provided robust protection against malaria in young adults.
Study Results
The study reported that out of nine participants who received the late-liver-stage attenuated malaria parasite, known as GA2, a staggering 89% (eight individuals) were successfully shielded from malaria infection. In stark contrast, the effectiveness of a previously developed early-arresting parasite (GA1) was much lower, with only one out of eight individuals (13%) achieving protection. Among three participants who received a placebo, all became infected, underscoring the potential impact of the GA2 vaccine.
Methodology and Findings
The trial, published in the New England Journal of Medicine, offers promising insight into a novel way of immunizing humans against malaria. This method relies on genetically modifying the Plasmodium falciparum parasite to stimulate an immune response while ensuring that the parasite dies before it can exit the liver and cause disease. Dr. Matthew McCall from Radboud University Medical Center elaborated that the GA2 parasite, which lacks a specific gene, survives for seven days in the liver before dying, while the earlier GA1 parasite has a much shorter lifespan of 24 to 48 hours.
Immune Response Enhancement
"This extended development time in the liver seems to enhance the immune response against malaria, which is the major takeaway from our study," said Dr. McCall.
Trial Structure
The trial consisted of two phases, with participants aged 19 to 35 between September 2021 and January 2022. In the first phase, participants were exposed to 15 or 50 mosquitoes infected with GA2 to assess safety. The second phase was a double-blind control trial comparing GA2's efficacy against GA1 and a placebo. Notably, no breakthrough infections occurred during the trial, with unpleasant side effects such as sore muscles and headaches reported equally across groups.
Controlled Infections
After three weeks of vaccinations, participants underwent controlled malaria infections via bites from mosquitoes carrying wild-type Plasmodium falciparum. All participants received treatment with a curative regimen after exposure, ensuring their safety. Interestingly, immune response analysis revealed that those inoculated with GA2 developed a more diverse set of immune cells, including a robust population of helper T cells, compared to those exposed to GA1.
Cautions and Future Directions
While the findings are globally promising, researchers caution that the trial's small sample size and controlled environment limit the scope of conclusions. Further studies, particularly in malaria-endemic regions, are essential to validate the vaccine's effectiveness and safety in populations at high risk of exposure.
Future Considerations
"In the future, we may need to look at administering this vaccine using needles rather than mosquito bites, for practical reasons," Dr. McCall noted.
Public Health Implications
As malaria continues to be a significant public health challenge, the emergence of such innovative vaccines could play a vital role in enhancing protective measures. Experts are keen to see future studies that could pave the way for expanded immunization strategies against malaria, especially for vulnerable populations. Stay tuned for more updates on this potentially game-changing development in the fight against malaria!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)