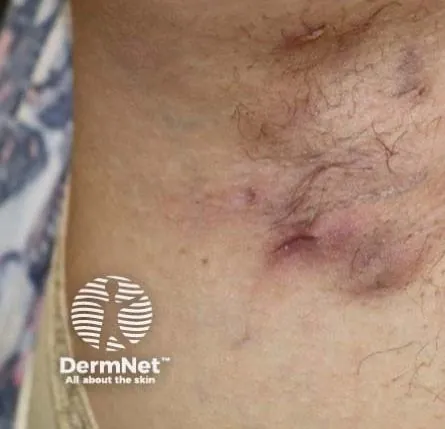
Secukinumab Shown to Alleviate Hidradenitis Suppurativa Symptoms and Enhance Mental Health Outcomes
2024-11-17
Author: Jia
Recent Findings from Phase 3 Trials
Recent findings from a comprehensive analysis of two pivotal phase 3 trials, SUNSHINE and SUNRISE, have shed light on the impressive benefits of secukinumab for patients battling moderate to severe hidradenitis suppurativa (HS). These results were presented at the Society for Dermatology Physician Associates' 22nd Annual Fall Dermatology Conference in Las Vegas, Nevada, and indicate a transformative impact not only on physical symptoms but also on accompanying psychiatric conditions.
Pooled Data Analysis
The pooled data, encompassing 1,084 patients, was meticulously analyzed, where participants were either given a placebo or treated with secukinumab at a dosage of 300 mg every two or four weeks for the initial 16 weeks. After this period, patients receiving placebo were allowed to switch to active treatment, offering everyone the chance to benefit from this innovative therapy. Notably, the group consisted predominantly of women (56.3%), with an average age of 36.2 years, all of whom exhibited significant HS symptoms prior to the trials.
Psychiatric Conditions and Efficacy
Previous evaluations of the trials highlighted secukinumab's sustained efficacy, overall enhancement in quality of life, and a strong safety profile. Remarkably, individuals with pre-existing psychiatric conditions were not excluded from the study, leading to revelations about the prevalence of depression and anxiety among participants. The rates of these conditions were noticeably higher than those reported in the general population, with depression observed at 9.8% compared to expectations of around 3.8%, and anxiety at 7.1% against a predicted 4%.
Impact on Mood and Emotional Well-Being
Further insights were gathered through the European QoL 5-Dimension (EQ-5D) questionnaire, which closely monitored mood and anxiety symptoms throughout the study duration. By the 16-week mark, a notable improvement in mood was recorded among patients taking secukinumab, which persisted even after 52 weeks. Those who transitioned from placebo to secukinumab also exhibited positive changes, suggesting that the medication could be beneficial regardless of treatment timeline.
Emotional Impact and Patient Assessments
The research team delved deeper into the emotional impact of HS on patients using the HS Symptom and Impact Diary (HSSID). When participants were asked to assess the effects of their condition on their emotional state, both treatment groups reported significant emotional disturbances; however, individuals in the secukinumab group showcased the most substantial improvement through the study's duration.
Psychiatric Adverse Effects
Concerning psychiatric adverse effects, the study reported relatively low incidence across all treatment groups. A few serious cases emerged, including two instances of suicidal ideation and one suicide attempt, all within a demographic of patients already diagnosed with depression prior to the study's commencement. Noteworthy symptoms emerged as depression (non-major), insomnia, and anxiety.
Conclusion and Future Implications
In conclusion, the findings underscore the profound benefits secukinumab can offer to patients coping with HS, particularly in fostering mental and emotional well-being. This comprehensive analysis suggests that the drug's holistic effect likely stems from its overall efficacy in alleviating HS symptoms. As new treatments emerge, the potential for enhancing quality of life for patients grappling with both physical and psychological challenges becomes increasingly evident. The results from these trials are promising, paving the way for improved therapeutic strategies in managing not only the skin condition itself but the emotional toll it takes on individuals.
Stay tuned—these findings may change the future of HS treatment and psychiatric care!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)