The Revolutionary Potential of Multimetallic Aerogels in Electrocatalysis
2024-12-09
Author: Ming
Introduction
Have you ever thought it was possible to transform heavy metals into an incredibly light aerogel? Surprisingly, this groundbreaking concept has been developed since its initial introduction by Eychmüller’s team back in 2009. The researchers showcased the creation of metal aerogels (MAs) by uniquely assembling metal nanoparticles, which opened the door to a vibrant field in materials science.
Promise of Multimetallic Aerogels (MMAs)
Among these novel materials, multimetallic aerogels (MMAs)—which incorporate more than one type of metal—have emerged as extremely promising candidates. The unique properties of MMAs arise from the synergetic interactions between different metals, providing a landscape of tunable characteristics that can significantly enhance electrocatalysis performance over traditional single-component MAs.
Performance and Modulation of MMAs
Research has shown that MMAs often outperform their single-metal counterparts. The secret lies in the ability to modulate their electrical conductivity, lattice parameters, and electronic structures by varying combinations of metals. This leads to a crucial question: how far can we push the boundaries of these material properties through controlled synthesis?
A Recent Study in Matter
A recent paper published in *Matter* titled "Manipulating Multimetallic Effects: Programming Size-Tailored Metal Aerogels as Self-Standing Electrocatalysts" dives deeper into this topic. It discusses how multimetallic effects can impact both the sol-gel process of metals and the resulting ligament sizes of MMAs.
Gravity-Driven Gelation Behavior
Five years ago, the team uncovered a novel, gravity-driven gelation behavior in metal systems, akin to a precipitation process. This discovery was pivotal in understanding how the high density of metals, such as gold (approximately 19.3 g/cm³), influences their settling in solution and the formation of monolithic gels.
Bimetallic Systems and Densities
Taking the exploration further, the researchers experimented with bimetallic systems, such as gold and silver. Results showed that the introduction of a lower-density metal like silver (around 10.5 g/cm³) effectively reduces the overall density of the metal aggregate. This alteration leads to a slower sedimentation rate, extending the gelation process and allowing for more nuanced control over material formation.
Controlling Ligament Size
The key takeaway from the study was the ability to control the ligament size of MMAs through multimetallic interactions. Ligament size is a critical factor influencing the material's physicochemical properties, and researchers have historically manipulated it through the use of initiators or ligands—which could inadvertently introduce contaminants. By contrast, the latest findings indicate that the deliberate introduction of auxiliary metals can significantly decrease ligament size, with reductions between 30% and 78% observed in various metal matrices.
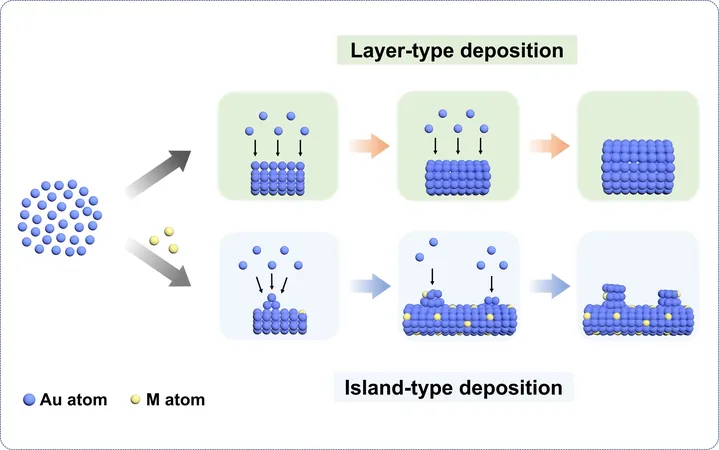
Atomic Radius Mismatch and Growth Types
This remarkable change is attributed to the atomic radius mismatch that occurs when different metals interact. Such mismatches can prevent conventional layer-type deposition, promoting instead an island-type growth that yields finer ligament structures, thus amplifying the electrophysical effects desired in catalysis.
Advancements in Electrocatalysis
In a notable advancement utilizing their gravity-driven gelation technique, the researchers developed a non-destructive sedimentation strategy that enhances the electrocatalytic performance of MMAs. By strategically placing carbon paper at the bottom of reaction vessels, the sedimentation of metal aggregates forms an integrated gel film which can directly function as a high-performing electrocatalyst for alcohol oxidation reactions.
Record-High Catalytic Efficiency
Remarkably, the Au-Pt gel film demonstrated record-high catalysis efficiency for both methanol and ethanol oxidation, showcasing the potential of these advanced materials to revolutionize electrochemical applications.
Conclusion and Future Prospects
In conclusion, this research not only sheds light on the manipulation of multimetallic effects in the design and structure of aerogels but also addresses long-standing challenges in producing robust electrocatalysts that exhibit high-performance metrics. The implications of such advancements could extend far beyond catalysis, paving the way for innovative applications in energy storage, environmental remediation, and smart material development.
Stay tuned for more breakthroughs as scientists continue to explore the fascinating world of multimetallic aerogels!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)