Alarming Warning: Unlicensed Medicines Could Lead to More Infant Deaths in England
2024-11-18
Author: Emily
Introduction
In a chilling wake-up call, a coroner has issued a stark warning that an increase in infant mortality linked to unlicensed medicines could occur in England if healthcare providers fail to transparently report complications associated with these treatments. This alarming conclusion follows a thorough inquest into the tragic deaths of three vulnerable infants due to contaminated nutrition.
The Tragic Cases
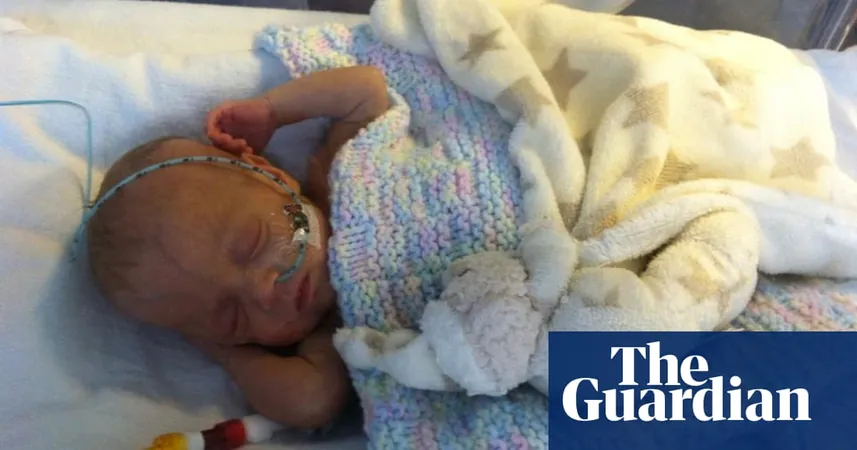
The inquest revealed that the babies—three-month-old Aviva Otte, one-month-old Oscar Barker, and nine-day-old Yousef Al-Kharboush—were receiving total parenteral nutrition (TPN) while being treated in hospitals after being born prematurely. Unfortunately, all three infants succumbed after being administered TPN tainted with Bacillus cereus, a harmful bacteria.
The Role of Healthcare Providers
This distressing saga unfolded when Aviva's mother, Jedidajah Otte, a journalist at The Guardian, highlighted her harrowing experience of being met with a "stubborn refusal" by medical staff at St Thomas' Hospital in London regarding her daughter's health status. Aviva tragically passed away in January 2014, having been provided TPN under a section 10 exemption of the Medicines Act 1968, which permits organizations to create bespoke medicines without the usual licensing for patients with specific needs.
Concerns Raised by the Coroner
In a twist of fate, Yousef and Oscar received their TPN from ITH Pharma, a licensed commercial supplier that avoids the constraints of the section 10 exemption. The coroner, Dr. Julian Morris, expressed deep concern over the lack of accountability for entities benefiting from this exemption, emphasizing that there is no obligation to report adverse events to regulatory authorities, such as the Medicines and Healthcare products Regulatory Agency (MHRA), or even to inform other healthcare trusts.
Ongoing Investigations and Legal Troubles
Dr. Morris elaborated on the risks associated with current reporting structures, suggesting a gaping hole in safety protocols that could potentially endanger fragile lives. Moreover, he underscored that Bacillus cereus is particularly resistant to common cleaning methods, necessitating rigorous decontamination processes that may not have been fully employed before the outbreak that led to these tragic deaths.
Call for Urgent Action
As further investigations unfold, urgent action is now required to address the highlighted risks to prevent any future fatalities. Recipients of the coroner's report are expected to respond by January 8, 2024.
ITH Pharma's Past Issues
In a separate incident that raises eyebrows, ITH Pharma faced legal troubles in 2022, resulting in a hefty £1.2 million fine after it was discovered that 19 premature babies had contracted infections due to inadequate safety measures during TPN provision across nine hospitals in 2014. The company admitted guilt to various regulatory offenses under both health and safety regulations and the Medicines Act related to the contaminated product.
Statement from ITH Pharma
In a statement, an ITH Pharma representative noted the potential benefits that shared information might have offered in preventing the catastrophic events of 2014, expressing sorrow and solidarity with the affected families. They emphasized their ongoing commitment to supporting NHS efforts to refine specialized feeding systems for the most vulnerable patients.
Conclusion
As the investigation continues, families are anxiously awaiting answers, grappling with the heartbreaking loss of their children while calling for necessary reforms to prevent such tragedies from occurring in the future. The health sector must now work collectively to ensure the safety of treatments provided to infants, highlighting the critical need for robust oversight of unlicensed medicines.
Stay Informed
Stay tuned for further updates on this pressing public health issue!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)