Breakthrough in mRNA Delivery: University of Pennsylvania Scientists Innovate with Advanced Lipid Nanoparticles!
2024-11-22
Author: Wei
Introduction
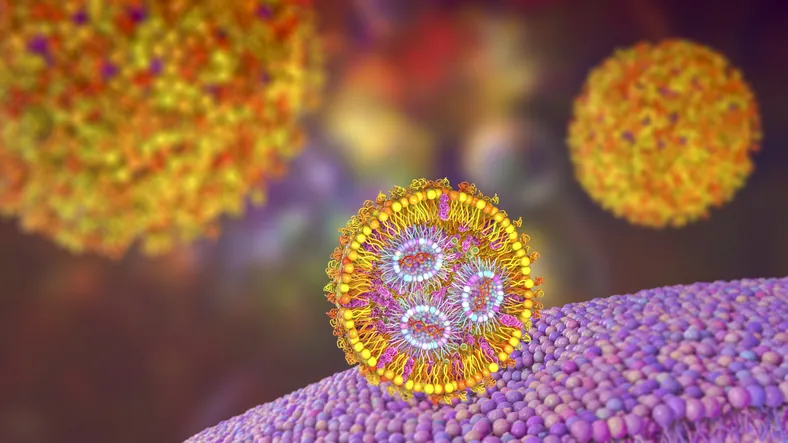
In an exciting development in the field of therapeutic delivery systems, scientists from the University of Pennsylvania have unveiled a revolutionary method for creating lipid nanoparticles that enhance the delivery of mRNA to targeted cells. Utilizing a groundbreaking approach known as A3 coupling, these researchers have engineered new lipid nanoparticles that outperform existing technologies, paving the way for more effective treatments for genetic disorders and infectious diseases.
A3 Coupling and A3 Lipids
The A3 coupling refers to the intricate amine–aldehyde–alkyne reaction that the Penn team expertly applied to fine-tune the structure of propargylamine-based ionizable lipids, leading to the creation of effective delivery vehicles that are both biodegradable and efficient. Known as A3 lipids, these innovative molecules self-assemble into lipid nanoparticles, which can navigate the body, securely carrying mRNA to its intended location, and release its contents without posing toxicity risks.
Performance of A3-Lipid Nanoparticles
Remarkably, the engineered A3-lipid nanoparticles exhibited superior performance in two critical applications: delivering genes to combat hereditary amyloidosis and enhancing the efficacy of the COVID-19 mRNA vaccine. According to studies published in Nature Biomedical Engineering, these novel A3 lipids significantly surpassed the current industry-standard lipids used for similar purposes, marking a crucial leap forward in mRNA delivery technology.
Directed Evolution for Lipid Optimization
In their recent article, titled "Optimization of the activity and biodegradability of ionizable lipids for mRNA delivery via directed chemical evolution," the team described their innovative use of directed evolution—a process involving five iterative cycles that allowed them to identify a plethora of biodegradable and asymmetric A3-lipids. These advancements were made in the context of establishing structure–activity relationships for lipid components, showcasing the meticulous nature of their research.
Significant Results
The results are astounding: the improved A3-lipid enhances the delivery of mRNA for genome editing and effectively transports an mRNA-based vaccine for SARS-CoV-2. The implications of their findings are vast, with potential applications extending to various mRNA-based vaccines and therapies designed to tackle genetic and infectious diseases.
Accelerated Development Timeline
What makes this method particularly promising is its potential to drastically reduce the time required to develop lipid nanoparticles. Traditional approaches to lipid development can take years, whereas the Penn team’s innovative directed evolution process promises to streamline this timeline to mere months or even weeks. Michael J. Mitchell, an associate professor in bioengineering at Penn, noted, "We hope this method will accelerate the pipeline for mRNA therapeutics and vaccines, bringing new treatments to patients faster than ever before."
Unique Properties of Ionizable Lipids
At its core, the success of these nanoparticles hinges on the unique properties of ionizable lipids, which can toggle between charged and neutral states. This transition is critical as it controls the nanoparticle's behavior in the bloodstream and inside target cells, ensuring safe and effective mRNA release.
Novel Approaches in Medicinal Chemistry
By ingeniously melding medicinal chemistry—which is generally meticulous but slow—with combinatorial chemistry known for its rapid synthesis capabilities, the Penn engineers have achieved a sweet spot of both speed and precision in the lipid development process. Research lead Xuexiang Han emphasized the creativity involved in stepping outside traditional methods to harness the full potential of the A3 reaction, which allows fine control over lipid molecular structures.
Conclusion and Future Implications
With this groundbreaking research, the University of Pennsylvania is poised to redefine how we approach mRNA delivery in therapeutics, and potential breakthroughs in medicine could be on the horizon. As the demand for advanced mRNA therapies and vaccines continues to grow, innovations like these will play a vital role in shaping the future of healthcare.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)