Unraveling the Link Between Blood Metabolites and Gastric Cancer: A Groundbreaking Study
2024-12-30
Author: Nur
Introduction
Gastric cancer (GC) continues to be a formidable opponent in the realm of global health, ranking fifth in cancer incidence and tragically sitting fourth in cancer-related mortality. In the wake of 2020, over 1 million new cases of GC surfaced globally, leading to nearly 769,000 deaths. Therefore, enhancing prevention and facilitating early detection of this deadly disease has become paramount.
Metabolic Alterations in Cancer
Recent research sheds light on the relationship between metabolic alterations and cancer, emphasizing that cancerous cells undergo significant metabolic reprogramming. This reprogramming equips these cells with the energy and resources needed to grow rapidly and survive in tough conditions. For GC, several metabolic pathways—including amino acid catabolism, lipid metabolism disruptions, and carbohydrate metabolism anomalies—have been implicated in both the initiation and advancement of the disease.
Biochemical Markers
One study observed a decrease in the amino acid tryptophan and an increase in phenylacetylglutamine levels in GC patients, hinting at possible biochemical markers for early detection. Furthermore, evidence suggests that tumor cells might be consuming fatty acids aggressively for energy and membrane synthesis, signaling a potential area for therapeutic focus.
Challenges in Observational Findings
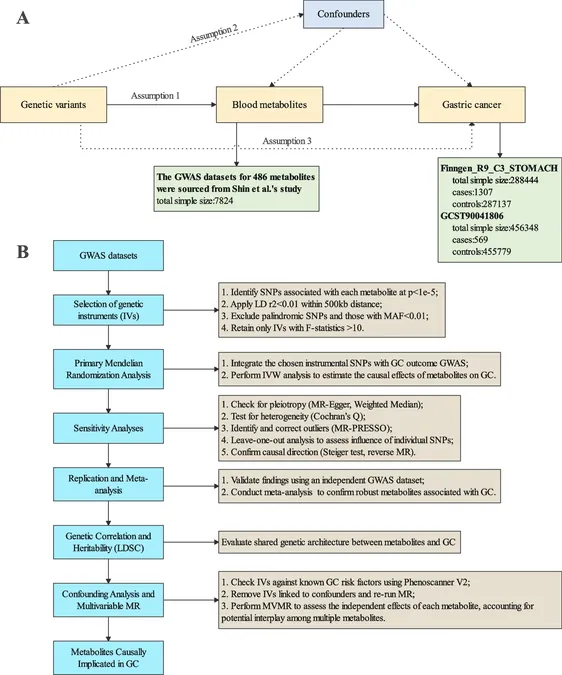
Yet, observational findings already known are often hampered by confounding factors and an inability to establish causation definitively. The integration of genetic data from genome-wide association studies (GWAS) allows researchers to explore how genetic determinants affect metabolite levels and interplay with diseases like GC. By utilizing Mendelian randomization (MR) analysis, researchers aim to clarify whether blood metabolites directly influence GC risk, bypassing many limitations of traditional observational studies.
Methodology of the Study
In this extensive study, investigators employed a two-sample MR design to evaluate the association between human blood metabolites and GC risk systematically. Utilizing data from GWAS, including 486 metabolites from 7,824 individuals in TwinsUK and KORA cohorts, they isolated independent genetic variants and confirmed robustness through several analytical strategies.
Key Metabolites Identified
The researchers identified critical metabolites potentially influencing gastric cancer risk: namely 3-methyl-2-oxovalerate, piperine, and the Phe-Phe dipeptide. Notably, elevated levels of 3-methyl-2-oxovalerate—linked to metabolism of branched-chain amino acids—were associated with an increased risk of gastric cancer. This compound's role may be essential not just in cancer progression but in cellular metabolism strategies favoring growth and energy production.
Piperine's Dual Nature
Piperine, an alkaloid derived from black pepper, showed dual aspects: while it had pro-cancer properties amid tumor models due to its impact on oxidative stress and inflammatory pathways, it has also garnered attention for its broader pharmacological properties.
Protective Associations of Phe-Phe Dipeptide
On the other hand, the Phe-Phe dipeptide, a lesser-studied entity, emerged with potential protective associations against gastric cancer, emphasizing the need for deeper investigations into its effects and mechanisms.
Conclusion
This research marks a seminal step in understanding the metabolic intricacies of gastric cancer pathogenesis. By meticulously analyzing the genetic associations and causal links between metabolites and cancer risks, the study illuminates not only potential biomarkers for early detection but also strategic therapeutic targets. As the cancer landscape continually evolves, the integration of metabolic profiles into public health strategies may revolutionize how we approach gastric cancer prevention and treatment. However, further research is needed to untangle these complex biochemical relationships and optimize their application in clinical settings.
Future Directions
So, could these blood metabolites be the key to unlocking new early detection tools and therapeutic avenues for gastric cancer? This high-stakes question looms as the global health community looks onward towards innovative solutions.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)